
Australia’s National Cervical Screening Program (NCSP) guidelines have been updated and now support the use of self-collected human papillomavirus (HPV) tests in more scenarios.
These include test of cure follow-ups after treatment of high-grade abnormalities.
Here is an overview of the most significant changes for primary care.
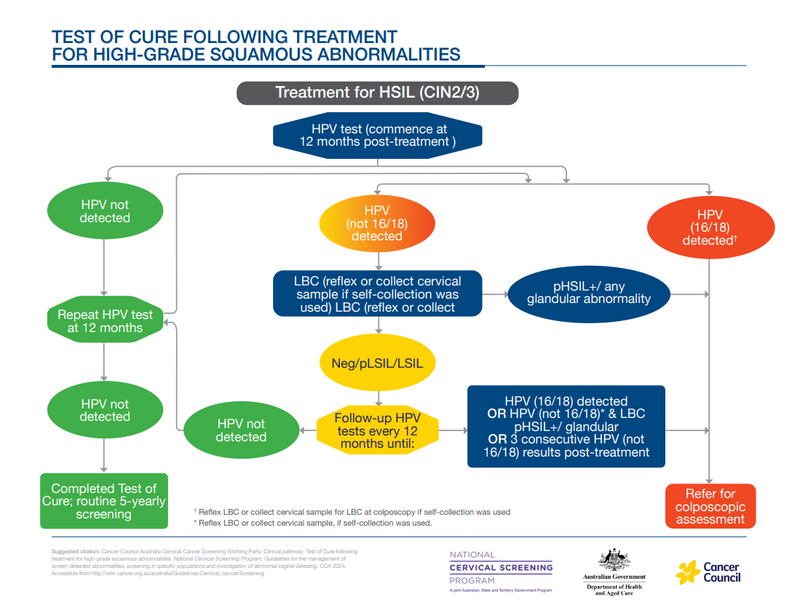
Post-treatment management (‘test of cure’) recommendations for people who have been successfully treated for a high-grade squamous intraepithelial lesion (HSIL)
People treated for HSIL are recommended to have annual HPV tests, rather than co-tests. When tests are negative on two consecutive occasions, they can return to routine screening.
If HPV not 16/18 is detected, the patient returns for liquid based cytology (LBC). If HPV 16/18 is detected they are referred for colposcopy. People with HPV not 16/18 detected on three consecutive annual post-treatment tests should be referred for colposcopy.
For more information, see 9.2.3 Test of Cure after treatment for HSIL (CIN2/3).
Post-treatment management recommendations for people who have been successfully treated for or adenocarcinoma in situ (AIS)
Follow-up annual co-tests after completely excised AIS can been extended to three-yearly testing if all tests have been negative for five years. They can cease 25 years after completely excised AIS if all tests have been negative.
If the person is aged less than 70, they can be returned to routine screening.
If the person has had at least one negative co-test when aged 70 or older, they can exit screening.
If any abnormal result is obtained on any follow-up co-test, the person should be referred for colposcopic assessment.
For more information see 9.3.2 Follow-up after completely excised AIS.
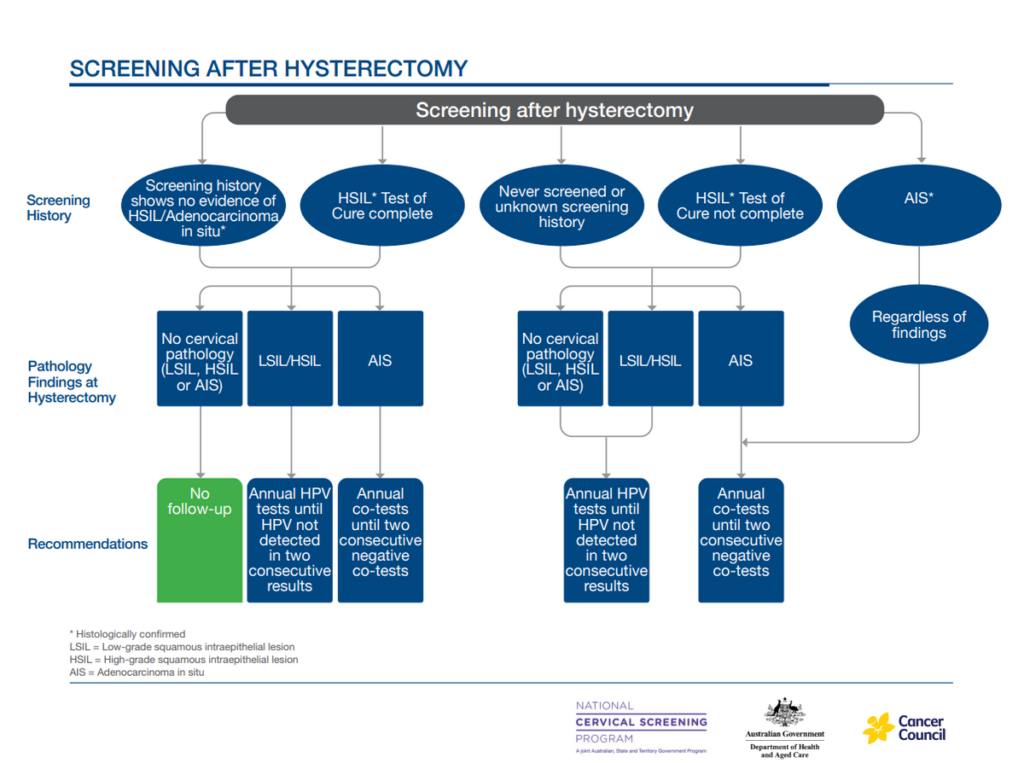
Where testing is indicated after a total hysterectomy
This has been simplified to annual testing, which can be co-testing or HPV testing, depending on cervical pathology and history, until oncogenic HPV is not detected on two consecutive occasions.
For more information, see 7.4 After total hysterectomy.
Non returners for LBC after non 16/18 HPV on self-collected sample
Screening participants with HPV (not 16/18) detected on a self-collected sample who do not return until nine months or more after the HPV test can be offered a follow-up self-collected HPV test, rather than liquid-based cytology (LBC).
This will determine if the HPV infection has now been cleared and the person can return to routine screening.
For more information, see Chapter 6.2 Oncogenic HPV types not 16/18.
Clarification of immune-deficient status
Categories of people for whom specific screening is needed due to immune-deficient status have been clarified and expanded.

Three-year screening is recommended for patients:
- Living with human immunodeficiency virus (HIV)
- Solid organ transplant with immunosuppressive therapy
- Active haematological malignancy
- Haematopoietic stem cell transplant (HSCT) recipients or chimeric antigen receptor T-cell (CAR-T) therapy within two years of transplantation.
- Primary immunodeficiency including combined immunodeficiency and syndromes, major antibody deficiency (e.g. common variable immune deficiency [CVID] or agammaglobulinemia), defects of innate immunity (including phagocytic cells), primary defects of immune regulation, complement deficiencies and phenocopies of primary immunodeficiencies.
Three-year screening should be highly considered for patients having:
- Long-term haemodialysis or peritoneal dialysis (>6 months)
- Long-term treatment (>6 months) with highly immunosuppressive therapies, including:
- high-dose corticosteroid treatment equivalent to >20 mg/day of prednisone for ≥14 days in a month, or pulse corticosteroid therapy
- selected conventional and targeted synthetic disease-modifying anti-rheumatic drugs (sDMARDS), taken for long-term treatment (> 6 months) including: mycophenolate, methotrexate (≥10 mg/week), azathioprine (≥1 mg/kg day), 6-mercaptopurine (≥0.5 mg/kg/day), alkylating agents (e.g.: cyclophosphamide, chlorambucil), and systemic calcineurin inhibitors (e.g. cyclosporin, tacrolimus), JAK inhibitors (e.g. tofacitinib, baricitinib, ruxolitinib, upadacitinib). Excluding: hydroxychloroquine or sulfasalazine when used as monotherapy or low-dose corticosteroid
- Biologic therapies that deplete T cells (e.g. muromonab, teplizumab) Excluding: other biologics that have other targets, for example anti-CD20 (e.g. rituximab) or anti-TNF (e.g. infliximab, adalimumab)
- Multiple immunosuppressants where the cumulative effect is considered to be severely immunosuppressive.
For more information, see Chapter 7.2 Immune-deficient people.
For more information about these updates to the National Cancer Screening Register see this page.
To access the full guidelines, go to this Cancer Council Australia page.





