
The Victorian Department of Health introduced the Respiratory Syncytial Virus Mother and Infant Protection Program (RSV-MIPP) in February 2025.
RSV is a virus that causes infection in the lungs and airways. It is one of the most common causes of respiratory infections in children.
Most cases are mild and can be treated at home, but babies and children can have complications of viral infections, including bronchiolitis, pneumonia and ear infections.
The RSV-MIPP is available free for pregnant women and eligible infants. It prioritises uptake of the maternal Abrysvo® RSV vaccine.
The vaccine is available under the NIP for all women at 28–36 weeks pregnancy. This reduces the risk of severe RSV disease in infants under six months by around 70 per cent. It can safely be given with other pregnancy-recommended vaccines such as those for influenza, pertussis and COVID-19.
To protect infants most at risk from severe RSV disease, the Department will soon implement a complimentary program with a free long-acting RSV monoclonal antibody, Beyfortus™ (nirsevimab).
This will run from 1 April to 30 September 2025. Early evidence from nirsevimab programs in the US and Europe have demonstrated real-world vaccine effectiveness of between 70 and 90 per cent.
Why has a mother and infant vaccination program been introduced?
RSV is a common infection. Almost all children will have it at least once within the first two years of life. Although most people experience a mild illness and recover in one or two weeks, RSV infections are especially serious for:
- infants aged 12 months or under
- young children or older adults with chronic health conditions
- Aboriginal and Torres Strait Islander infants.
In 2024 there were over 250 deaths in Australia from RSV infection. Between 2016 and 2019 there were more than 115,000 hospitalisations with an RSV diagnosis. Most of these were for children aged less than five who were otherwise healthy.
The annual RSV hospitalisation rate for infants under six months over the same period was approximately 6,200 per 100,000 population. The highest rates – 7,200 per 100,000 – were in infants aged two months or under.
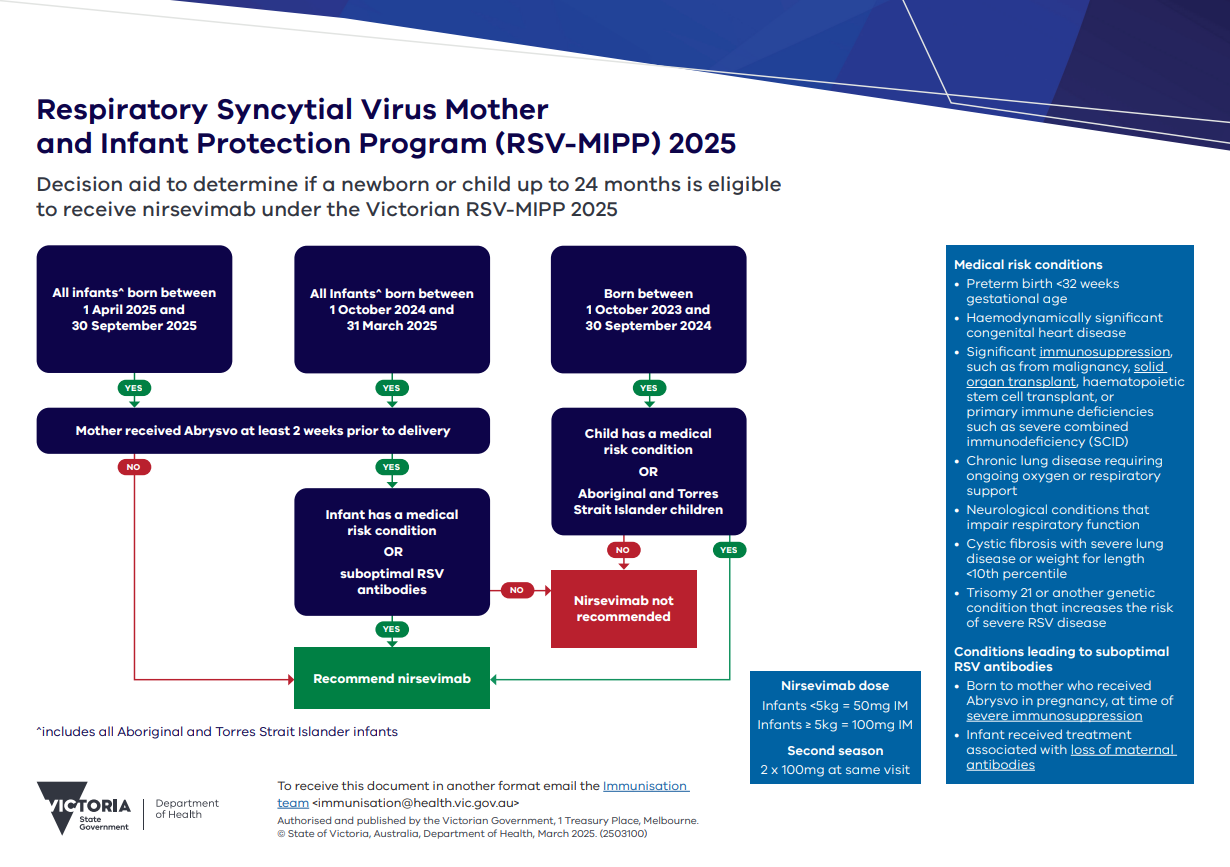
Victorian infant immunisation program 2025 – eligibility
Neonate program
Infants born between 1 April – 30 September 2025:
- To mothers who did not receive maternal RSV vaccine during pregnancy, or where maternal RSV vaccine was administered fewer than two weeks before birth
- with risk conditions for severe RSV disease (see the Australian Immunisation Handbook for specifics) regardless of maternal vaccination.
Catch up program – first RSV season
Infants born from 1 October 2024 – 31 March 2025:
- For infants up to eight months only, to mothers who did not receive maternal RSV vaccine during pregnancy, or where maternal RSV vaccine was administered two weeks before birth, or
- with risk conditions for severe RSV disease (see the Australian Immunisation Handbook for specifics) regardless of maternal vaccination.
Catch up program – second RSV season
Infants born on or after 1 October 2023 vulnerable to severe RSV:
- Aboriginal Torres Strait Islander infants
- Young children with conditions associated with increased risk of severe RSV disease (see the Australian Immunisation Handbook for details).
What are the expected vaccine side effects?
Both Abrysvo® and Beyfortus™ (nirsevimab) are safe and have been approved by the Therapeutic Goods Administration.
Abrysvo® RSV vaccine for pregnant women
Mild common side effects could occur up to 40 per cent of recipients. They are:
- pain and redness at the injection site
- fatigue
- headache
- muscle pains.
Rare side effects include allergic reactions, and two possibilities currently being monitored:
- Guillain-Barré Syndrome (GBS): this might occur more often than expected after an RSV vaccine. The rate found in USA studies was around 4.6–9.5 cases per million doses in the first 1–21 days after vaccination, compared to the usual rate of two cases per million doses. However, GBS is still very rare, and more research and monitoring are needed to confirm these findings. Influenza vaccine also has a small risk of increasing GBS cases, around 1-2 per million vaccine doses.
- Premature births: a clinical trial of a now-discontinued GSK RSV vaccine for pregnant women found more premature births and newborn deaths in vaccinated women compared to women who had not received the vaccine, mostly in low- and middle-income countries. Because of this, rates of premature births in women who receive any RSV vaccine are being actively monitored. In trials for the Abrysvo vaccine, there is no clear evidence of an increase in premature births among women who had received the vaccine. Until more data are available, it is recommended that as a precaution, the Abrysvo vaccine only be given to women who are at least 28 weeks pregnant.
Beyfortus™ (nirsevimab) monoclonal antibody for infants
Minor side effects occur approximately one per cent of the time. They are:
- pain, redness and swelling at the injection site
- rash.
Rare side effects seem to be limited to allergic reactions. No rare adverse events are being monitored.
How will I know if an infant has already had a dose of Beyfortus™ (nirsevimab) at birth?
Supply of Beyfortus™ (nirsevimab) will be limited so it is important to avoid double-ups where possible. Many local maternity units do not upload to the Australian Immunisation Register (and as this is not a NIP vaccine they are not obliged to) so reviewing the green book when infants are presenting for vaccines will be essential.
How will I identify which infants to recall for Beyfortus™ (nirsevimab)?
There is no dedicated PENCAT recipe to recall infants for RSV vaccine yet. However, it is possible to generate an invitation list based on active patients in the correct age range for first RSV season recalls. For second RSV season recalls you could use patients in the correct age range and Indigenous status. Unfortunately, none of the high-risk conditions are identifiable on PENCAT.
Can I write a private prescription for Beyfortus™ (nirsevimab) for infants who do not qualify for the funded program?
At the time of writing there is no private supply of Beyfortus™ (nirsevimab).
For more information:
- Victorian Department of Health | RSV immunisation
- Australian Immunisation Handbook | Respiratory syncytial virus (RSV)
- Australian Immunisation Handbook | Flowchart for which infants should receive nirsevimab in their first RSV season
- National Centre for Immunisation Research and Surveillance Australia | RSV FAQs
- NSW Health | Maternal RSV vaccination (Abrysvo®) fact sheet
- Health Pathways | Immunisations (pregnancy)
- Health Pathways | Immunisations (childhood)





